electron configuration of cadmium|Chemistry of Cadmium : Bacolod Cadmium, a transition metal, has the chemical symbol of Cd. Cadmium is located in the d-block and 12 group of the periodic table possessing an atomic number of 48 and an atomic mass of 112.411g. . Police Station 4, National Highway, Olongapo City, Zambales. Gandara Pcp. Sabino Padilla Street, City of Manila, Metro Manila. Nearby Businesses. 0.08 km. Saver's Appliance Depot. 1200 Rizal Avenue, East Tapinac, Olongapo City 2200 Zambales. 0.08 km. Jackya Travel Services.Watch Sa Gubat Dinonselya Ang Masikip na Kweba ni Maria (Iyot sa Pwit) on iyotFlix.com, the #1 Porn site.
PH0 · How to Write the Electron Configuration for Cd and Cd2+
PH1 · Electron configuration of Cadmium
PH2 · Complete Electron Configuration for Cadmium (Cd, Cd2+)
PH3 · Chemistry of Cadmium
PH4 · Cadmium electron configuration
PH5 · Cadmium Electron Configuration (Cd) with Orbital
PH6 · Cadmium (Cd)
PH7 · Cadmium
PH8 · 11.2: Chemistry of Cadmium
ESO Turns 10! Get ready for reveals, retrospectives, and rewards as we celebrate 10 incredible years of The Elder Scrolls Online over a massive 15-month long period.. On this page, you can discover all the latest Anniversary Celebration news, review upcoming in-person events, and learn more about the game, its developers, and the community that .
electron configuration of cadmium*******The ground-state electron configuration of cadmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. This electron configuration shows that the last shell of cadmium has two electrons and the d-orbital has a total of ten electrons. Therefore, the valence electronsof cadmium are two. The . Tingnan ang higit pa
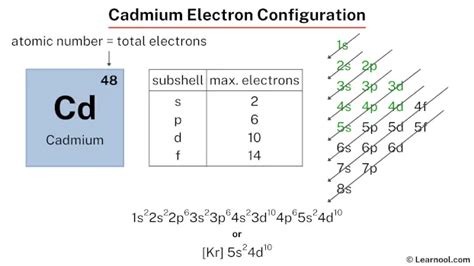
The total number of electrons in cadmium is forty-eight. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit paChemistry of Cadmium Cadmium, a transition metal, has the chemical symbol of Cd. Cadmium is located in the d-block and 12 group of the periodic table possessing an atomic number of 48 and an atomic mass of 112.411g. .
Electron configuration of Cadmium. The electron configuration of Cadmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. The chemical element located in group 12 of the . The cadmium electron configuration, denoted as [ Kr] 5s 2 4d 10 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10, showcases the specific placement of electrons within the atom. This configuration can .
Cadmium, a transition metal, has the chemical symbol of Cd. Cadmium is located in the d-block and 12 group of the periodic table possessing an atomic number of 48 and an atomic mass of 112.411g. . What is the Electron Configuration of Cadmium? Kr 4d10 5s2 is the electron configuration of Cadmium. How Many Valence Electrons Does Cadmium Have. There are two valence electrons in the outer shell . To write the configuration for the Cadmium and the Cadmium ion, first we need to write the electron configuration for just Cadmium (Cd). We first need to find the .The known isotopes of cadmium range in atomic mass from 94.950 u ( 95 Cd) to 131.946 u ( 132 Cd). For isotopes lighter than 112 u, the primary decay mode is electron capture and the dominant decay product is .
Cadmium is a transition metal with symbol Cd and atomic number 48. Its electron configuration is [Kr] 4d 10 5s 2, with two valence electrons. Learn more about its .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .
Cadmium Electron Configuration: Cadmium is a chemical element which has a chemical symbol Cd. The atomic number of the Cadmium is 48. It is soft, bluish-white metal and is chemically similar to .
Electronic configuration of the Cadmium atom. Valence electrons. Orbital diagram. Cadmium electron configuration. ← Electronic configurations of elements . Cd (Cadmium) is an element with position number 48 in the periodic table. Located in the V period. Melting point: 321 ℃.Cadmium is a chemical element of the periodic table with chemical symbol Cd and atomic number 48 with an atomic weight of 112.414 u and is classed as a transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 5s 2: Electrons per shell: 2, 8, 18, 18, 2: Valence electrons : 2: Valency electrons : 2:Abbreviated electronic configuration of Cadmium. The ground state abbreviated electronic configuration of Neutral Cadmium atom is [Kr] 4d10 5s2. The portion of Cadmium configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Kr]. For atoms with many electrons, this notation can become lengthy . Your starting point here will be the electron configuration of a neutral cadmium atom.. Cadmium, #"Cd"#, is located in period 5, group 12 of the periodic table and has an atomic number equal to #48#.This means that a neutral cadmium atom will have a total of #48# electrons surrounding its nucleus.. This also tells you that the . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait . Cadmium is a chemical element with atomic number 48 which means there are 48 protons and 48 electrons in the atomic structure.The chemical symbol for Cadmium is Cd. Electron Configuration and Oxidation States of Cadmium. Electron configuration of Cadmium is [Kr] 4d10 5s2. Possible oxidation states are +2. Electron .
Cadmium, complete electron configuration. © 2009-2016 | www.prvky.com | kontaktkontakt
The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in . All three elements in group 12 have ns 2 (n − 1)d 10 valence electron configurations; consequently, the +2 oxidation state, corresponding to losing the two ns electrons, dominates their chemistry. In addition, mercury forms a series of compounds in the +1 oxidation state that contain the diatomic mercurous ion Hg 2 2 + .
Die Elektronenkonfiguration von Cadmium ist 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. Das chemische Element in Gruppe 12 des Periodensystems heißt Cadmium, seine Ordnungszahl ist 48 und das Symbol dieses Elements ist Cd. Cadmium atoms have 48 electrons and the shell structure is 2.8.18.18.2. The ground state electron configuration of ground state gaseous neutral cadmium is [Kr].4d 10.5s 2 and the term symbol is 1 S 0.
electron configuration of cadmium Chemistry of Cadmium 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^"10" 4p^6 4d^"10" or [Kr] 4d^"10" The atomic number of cadmium, Cd, is 48. Thus, the ground state electron configuration of this element is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^"10" 4p^6 5s^2 4d^"10" or [Kr] 4d^"10" 5s^2 But since the problem is draw the electron configuration of the Cd^"2+", this .
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .
Its electron configuration is \[\ce{He}:\, 1s^2 \nonumber \] The three electrons for Li are arranged in the 1s subshell (two electrons) and the 2s subshell (one electron). The electron configuration of Li is \[\ce{Li}:\, 1s^22s^1 \nonumber \] Be has four electrons, two in the 1s subshell and two in the 2s subshell. Its electron configuration is 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration. Na has the same electron configuration as Ne with the addition of 3s 1. Na's noble gas configuration is [Ne]3s 1.镉的电子排布为 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2。 位于元素周期表第 12 族的化学元素称为镉,其原子序数为 48,该元素的符号为 Cd。
CSS (Cascading Style Sheets) is the code that styles web content. CSS basics walks through what you need to get started. We'll answer questions like: How do I make text red? How do I make content .
electron configuration of cadmium|Chemistry of Cadmium